


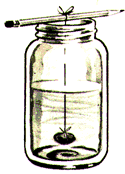
In a jar add to very hot water whichever substance you are working with until saturation is reached. Pour the solution off into a clean jar, leaving behind any undissolved substance.
Suspend a thin thread into the center of the jar. The thread can be tied to a pencil, Popsicle stick, or whatever is handy and will span the jar opening. Alternately, you can punch a hole in the lid of the jar, pass the thread through the hole, and then use a nail or pencil to hold the thread in place from the outside.
 If you are using the jar lid, screw it on, and stick a piece of masking tape over the hole. If you are not using the jar lid, tape a piece of paper over the mouth of the jar. This is to control the rate of evaporation. Let sit, then after 15 minutes, swish the jar a bit. Swish it again 15 minutes later, then one final time an hour later. Set the jar where it won't be disturbed. Depending on the substance used, the crystals should begin to grow in an hour or so, and continue to grow for from a day to several days.
Old-fashioned rock candy is grown using basically the same setup as above. Sugar is used for the solution, and the crystals are typically grown on a wooden stick rather than a thread.
If you are using the jar lid, screw it on, and stick a piece of masking tape over the hole. If you are not using the jar lid, tape a piece of paper over the mouth of the jar. This is to control the rate of evaporation. Let sit, then after 15 minutes, swish the jar a bit. Swish it again 15 minutes later, then one final time an hour later. Set the jar where it won't be disturbed. Depending on the substance used, the crystals should begin to grow in an hour or so, and continue to grow for from a day to several days.
Old-fashioned rock candy is grown using basically the same setup as above. Sugar is used for the solution, and the crystals are typically grown on a wooden stick rather than a thread.
With a little more effort it is possible to produce a single large symmetrical crystal. First fill a jar with hot water, and stir in as much salt as will dissolve. This is a supersaturated solution. Allow the excess salt to settle out, and pour the mixture into a saucer, leaving all of the undissolved salt behind.
As the water cools, tiny crystals will begin to form on the bottom of the saucer. These are the "seed" crystals. Using a magnifying glass, pick out the largest perfectly formed crystal. Hold on to any well shaped seed crystals in case your first attempt does not go well.
Mix up another batch of hot supersaturated solution. Allow the undissolved salt to settle out as before, and pour off into a clean jar. You can use a coffee filter or clean napkin as a filter. Tie a thin thread to the seed crystal and hang it in the solution. Cover the jar with a piece of paper to slow evaporation.
As the water evaporates over the course of several weeks, the salt will attach to the growing crystal. Remove any other crystals as they form. From time to time, you should add more cooled, supersaturated solution. Don't let the jar get too warm, as this may dissolve the crystal. Crystals formed this way tend to be very fragile, and of course will be dissolved by water.
This can be tried with other substances, such as bicarbonate of soda, alum, copper sulfate, photographer's hypo, borax, laundry soda, etc.
Heat one cup (250 ml) of water to boiling. Stir in about 3 tablespoons (45 ml) of alum until it dissolves. Pour the solution into a clean jar. Cover with plastic wrap or wax paper and set in a place where it will not be disturbed for several days. If the solution was pure enough, you should end up with a single large crystal.

Dissolve Epsom salts (magnesium sulfate) in a pot of boiling water until no more will dissolve. Add a drop or two of white glue (not critical). Brush a small amount of the liquid onto a glass or bottle. The liquid will quickly evaporate, leaving behind a film of delicate crystals.

Stalactites are the long rock columns that grow from the roofs of many caves. Stalagmites are similar columns that grow from the cave's floor. They are made from the minerals in water that drip down through the cave. It is possible to simulate the growth of these formations.
Fill two jars with very warm water. Add as much baking soda or Epsom salts to each jar as will dissolve. Place a saucer between the two jars, which are about a foot apart. Dip one end of a piece of absorbent (cotton, wool - some synthetics won't draw the solution) yarn, thick thread or string into each jar. The ends should be weighted with washers, nails or what have you to keep them in the jars. Let it hang in the middle, over the saucer. Capillary action should draw the baking soda solution up through the yarn, where it will drip onto the saucer. Over the course of several days, the dripping water will deposit the baking soda, forming a tiny stalactite and stalagmite. Eventually these may join to create a single column, as in an actual cave. Epsom salts generally take longer, but yield more variety of shape.

This article was printed from the Bizarre Labs website at bizarrelabs.com