



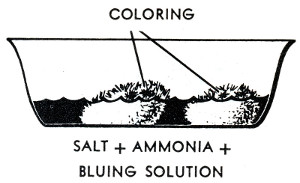
The crystal garden, coal garden, poor man's flower, chemical flower garden, Depression plant or Depression flower, as it was known at various times, is a classic science activity dating to the 1930s and probably earlier. The traditional recipe uses a mixture of table salt, Mrs. Stewart's liquid laundry bluing, ammonia and water poured over a porous substrate, often charcoal briquettes. There were similar and perhaps earlier projects using ammonium chloride and no bluing.(Hunter, Lockwood)
The crystal garden originated, or at least gained popularity, in the early 1930s, hence the contemporaneous names "Depression flower" and "Depression garden". (Thies) Around this time the Mrs. Stewart's company first provided instructions for making a chemical garden using their product.
Crystal gardens were something of a fad in the 1930s. Chemist Tenney L. Davis wrote in the february 1933 issue of Technology Review that
"Depression flower gardens which have recently appeared in so many homes, which are being discussed in business offices, in the newspapers, and at teas where the ladies forgather, may be prepared by mixing:
- 6 tablespoonfuls of salt
- 6 tablespoonsful of bluing
- 6 tablespoonfuls of water
- 1 tablespoonful of ammonia water
and pouring the mixture, after thorough mixing, over a clinker in a suitable dish. Not quite all of the salt will dissolve, but let the undissolved portion be poured out along with the rest. Instead of a clinker, a piece of coke, or of common brick, or a mass of coal ashes may be I used, but a clinker has the best degree of porousity and is probably best. After the clinker has been wet with the liquid, drop on it a few drops of mercurochrome solution or of red or green ink, or of any other colored liquid which is handy. But do not use iodine, for this reacts with ammonia water to form the dangerously explosive nitrogen iodide, a black powder which is safe so long as it is wet, but exlpodes with a loud report from a very slight shock when it is dry. Within 10 or 15 minutes after the materials have been brought together, a coral-like growth begins to appear on the clinker. This increases rapidly; within a few hours the clinker is entirely covered with a growth which is colored in part by the bluing and in part by the mercurochrome or other color which was used. The color of the bluing fades in time and changes to brown because of a chemical reaction with the ammonia. The colors produced by aniline dyes, ink, mercurochrome, and so on are permanent.
The growth also tends to form on the edges of the dish and will climb up and over them unless they have been rubbed with Vaseline. The growth will not extend beyond the Vaseline.
The "depression flower garden" is a capillary phenomenon involving the tendency of ammonium salts to "creep." The saturated solution deposits crystals around its edges and upon the clinker where the evaporation is the greatest. The crystals are porous, and act like a wick, sucking up more of the solution by capillary action. The solution thus sucked up evaporates to produce more crystals, more wick, and more growth. The addition of a little more ammonia water to the dish will produce more growth after the first growth has stopped. Or the whole may be allowed to dry and may then be kept without further change.
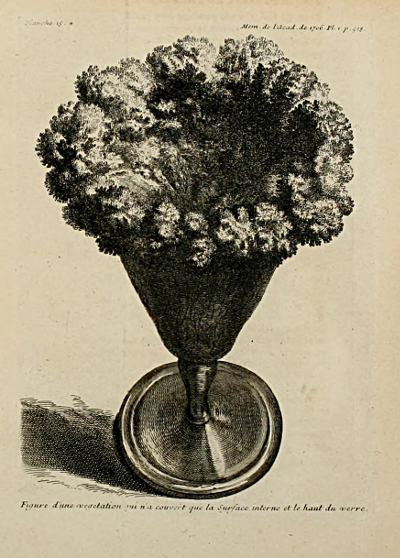
The idea of the "depression flower garden" is by no means new. In the years 1705, 1706, and 1707, the chemist Nicholas Lemery published in the memoirs of the French academy papers in which he described "vegetations" produced by the spontaneous evaporations of salt solutions. His papers were accompanied by engravings...Lemery secured his vegetations by the use of salts of iron and wrongly concluded from his experiment that iron is therefore necessary for the growth of plants.
One who makes a depression flower garden might be tempted to infer that ammonia is necessary for the growth of plants. This would be a wrong inference from the experiment, but it would be a correct judgement, for it is true that ammonia is essential to the growth of nearly every kind of vegetable organism."
Davis apparently confused French chemist Nicolas Lemery (1645 - 1715) with his son, Louis Lémery (1677-1743), also a chemist. Papers written by Lémery (or Lemery) on the subject are signed "Par M(onsieur)Lemery le fils (the son)", indicating that the author was Louis Lémery.
Davis, in the October 1933 issue of the Journal of Chemical Education, again mentions Lémery's experiments with “chemical vegetation” and included one of his illustrations. Lémery used iron filings, oil of nitre and oil of tartar to grow "chemical vegetation" to prove that iron could easily travel up the “pipes” of plants. As Davis said, Lémery “secured his ‘vegetations’ by the use of iron salts, and inferred that iron is therefore necessary to the growth of plants. A non-chemist at present, using ammonia water in the preparation of a ‘depression plant’, might similarly be tempted by a wrong process of inference to reach the right conclusion that ammonia is necessary to the growth of plants”.
Lémery’s paper on the subject that was printed in the 1706 Memoires de Mathematique et de Physique de l'Académie Royale des Sciences included these two illustrations, perhaps the earliest of a “chemical garden”. The first caption reads “Figure d'une végétation qui n'a couvert que la Surface interne et le haut du verre (figure of a vegetation that covered only the inner surface and the top of the glass)”, the second “Figure d'une autre vegetation plus belle et plus distincte qui S'est faite en beaucoup moins de tems que la premiere et qui a couvert le dedans et de dehors du verre et mesme une bonne partie du petit vaisseay qu'on a este oblige de mettre dessous (figure of another more beautiful and more distinct vegetation which was made in much less time than the first one and which covered the inside and outside of the glass and even a good part of the small vessel which one was obliged to put under it)”.

A more modern examination of the chemistry involved was presented at the Thirty-Second Annual Meeting of the North Carolina Academy of Science by O. J. Thies, Jr., and printed in Volume 19, Issue 1 of the Journal of the Elisha Mitchell Society.

Different formulae for growing crystal gardens have been printed over the years. All include table salt - non-iodized is often recommended - and liquid bluing. Most include ammonia, though later versions of the instructions provided by Mrs. Smith's leave it out due to health and environmental concerns. Ammonia, when used, must be the plain type, not the "sudsy" type.
There are fewer brands of bluing being made than there once were and Mrs. Stewart's is apparently the only one still being made that works for a crystal garden. It contains Prussian blue, Fe7(CN)18, or, more specifically, a soluable form KFeIIIFeII(CN)6 and an insoluable form FeIII4[FeII(CN)6]3·xH2O. The salt, bluing, ammonia and water are brought to the surface of the charcoal (or whatever porous material is used). Crystals form as the liquid evaporates. Some sources indicate that the crystals are simply salt re-crystalizing around an iron "seed" from the bluing with the ammonia only acting to hasten evaporation. The process is actually more complicated and involves the ammonia as well as the bluing and salt, forming (likely) ammonium chloride (NH4Cl) and two forms of ferrous ferrocyanide, KNaFeIIFeII(CN)6 and Na4FeII4[FeII(CN)6]3.
The bluing, salt, ammonia, and water are mixed in varying ratios depending on the recipe. Sometimes the water is left out altogether. Several of the more common recipes are given below.
Many of the instructions printed over the years specify non-iodized salt. In tests both seem to work just as well. So don't buy special salt just for this; any table salt should work.
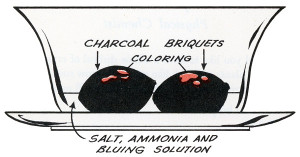
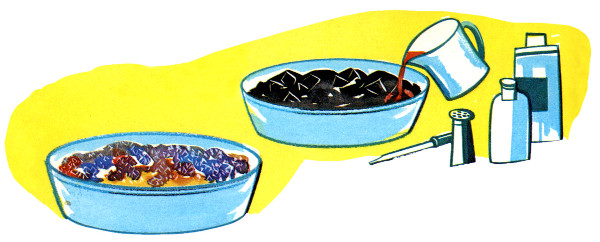
Charcoal is most often used these days, but several porous and readily available (at the time) materials have been suggested over the years. Coal was once more common in households and worked well, as did coke (a solid fuel made from coal), clinkers (a byproduct of burnt coal), porous bricks, and even bits of sponge. Natural charcoal is more porous and will work faster and usually produce larger crystals than compressed charcoal, but compressed charcoal will absorb more food color and consequently produce more colorful crystals.
A glass or ceramic container is usually recommended. A small glass bowl or a glass pie pan work well. Use something that you don’t mind staining blue. Plastic will work just as well. Metal is reactive and should be avoided.

Some sources (Stone, Saunders, Herbert, Challand) recommend
while others (Beeler) match Tenney L. Davis' slightly different ratios
The procedure is the same either way. Coal, coke, charcoal, or sponge, usually broken into smaller pieces, is spread evenly in a bowl or glass pie pan. It will work with whole briquettes but breaking them up makes a shorter pathway for the chemicals to travel and creates more surface area for crystals to form. Natural charcoal, being more porous, is recommended over compressed briquettes, but either will work so used what’s on hand.
Salt is dissolved in the water first. It helps if the water is hot. Not all of the salt will dissolve. Mix in the bluing and ammonia, then pour over the charcoal in the pan or bowl. The charcoal should not be completely submerged; halfway or less is recommended.
The crystals will be white unless color is added. The usual method these days is to add a few drops of food color here and there. Some books suggest colored inks or mercurochrome. Iodine should not be used; the results can be explosive!
Set the container somewhere relatively warm where it won't be disturbed. Somewhere the cat won’t get into it. Crystals may appear in a half hour or it may take a day or two. Be patient, they will grow! And they will continue to grow as long as there is still some liquid in the bottom of the container. You can keep them growing for a while by mixing more of the solution and pouring it in the bottom of the dish. Be careful not to pour it on top of the crystals.
Once dried out the crystals are very delicate and will fall apart if handled. Which is fine because now you can start the process over!

Some instructions call for the same amount of everything – one part salt, bluing, water and ammonia. Which is easy to remember and works well. But that’s really more ammonia than is needed. You'll likely have better results with 1 part ammonia and 3 or 4 parts everything else. Results will vary, so experiment!
Mrs. Stewart’s leaves out the ammonia in their instructions these days, presumably to make it a safer project. You may see a few salt crystals without the ammonia but they will not be the same fluffy crystals of yore, and certainly not as many. If you want big fluffy crystals, you're going to have to use the ammonia.
Soaking or wetting the charcoal with water, which is sometimes recommended, does not help, and sprinkling salt on the surface seems to have little effect.
Also some recipes leave out the water. In my experience, crystals form slower and are not as big.
Beeler, Nelson F. and Franklyn M. Branley. Experiments in Science. New York: Thomas Y. Crowell, 1955.
Challand, Helen and Elizabeth Brandt. Science Activities From A to Z. Chicago: Children's Press, 1963.
Cleveland, Robert. Cappy Dick's Pastime Book for Boys and Girls. New York: Greenberg, 1946, p. 156.
Davis, Tenney L. "Chemical Flower Gardens". Technology Review 35, February 1933, pp. 181-182.
Herbert, Don. Experiments For Young Scientists. Garden City, New York: Doubleday, 1959.
Hunter, George William and Walter George Whitman. Science in Our World of Progress, vol. 3. New York: American Book Company, 1935, p. 119.
Lockwood, Elizabeth Ann. A Study of the Teaching of General Science in Detroit, Michigan, with Particular Consideration of Enriching the Activities of the 7th, 8th, and 9th Grades. Cornell University, 1938, p. 144.
Saunders, John R. The Golden Book of Nature Crafts. New York: Golden Press, 1958.
Stone, George K. and Lucy W. Stephenson. Science You Can Use. Englewood Cliffs, New Jersey: Prentice-Hall, 1964.
Thies, O. J., Jr. "Notes on the 'Depression Plant'." Journal of the Elisha Mitchell Scientific Society, September 1933, p 15.
From top to bottom: Saunders, Stone, Challand, Mrs. Stewart's, Lémery, Lémery, Herbert, Saunders, Bizarre Labs.
This article was printed from the Bizarre Labs website at bizarrelabs.com